|
VoLPs: V order Logarithmic Polynomials
A new set of mathematical
functions, that is able to describe gases’ thermodynamic properties, has
been developed. These functions have the form of a fifth order logarithmic
polynomial (VoLP). They could be utilized for
combustion processes, with “frozen composition” and “composition in
equilibrium” evaluation. The VoLPs present
several advantages: they are able to cover a wide range of temperatures with
only a single mathematical function; they have an elevate accuracy and they
present the possibility to extrapolate experimental data beyond the
experimental temperature range. The VoLP
coefficients have been evaluated through the least squares fit on the basis
of experimental measurements (taken from scientific literature). The set of
VoLPs gives the possibility to study the
combustion phenomena and allows to describe specific heat at constant
pressure, enthalpy, entropy and equilibrium constants for gases
dissociation.
Specific heat at constant pressure:
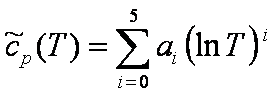 [J/(mol
K)] [J/(mol
K)]
Enthalpy:
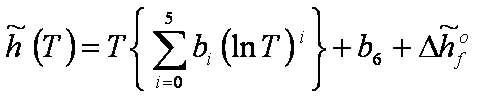 [J/mol] [J/mol]
Entropy:
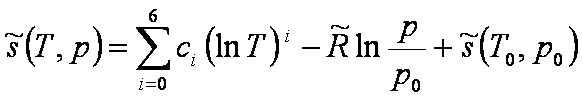 [J/(mol K)] [J/(mol K)]
Gibbs function:
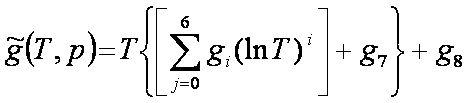 [J/mol] [J/mol]
Equilibrium constant (Kp) of a
thermo-chemical dissociation reaction:
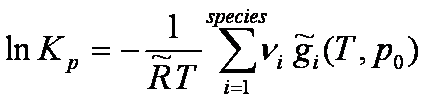 [-] [-]
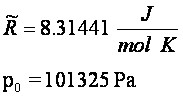
(with the convention that ni
is negative for the dissociation reaction reactant species)
Example: for the dissociation
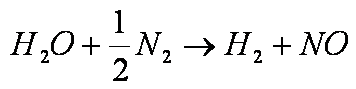
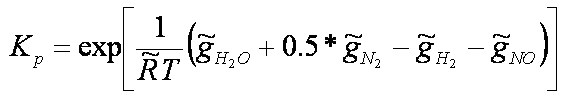
coefficients
|
Species
|
a5
|
a4
|
a3
|
a2
|
a1
|
a0
|
|
O2
|
0.06180721
|
-1.91808230
|
23.18329753
|
-135.04796311
|
377.22447890
|
-373.49246047
|
|
O
|
0.07027490
|
-2.31335083
|
30.19191662
|
-194.76523964
|
618.57117075
|
-748.19500164
|
|
N2
|
0.21448719
|
-7.30576818
|
97.95623827
|
-645.21170218
|
2087.25937009
|
-2624.96917520
|
|
N
|
0.14239267
|
-4.53379847
|
57.24529880
|
-358.12331558
|
1109.54271743
|
-1340.57218387
|
|
H2
|
-0.1571988
|
5.07583945
|
-64.62974617
|
406.63764375
|
-1265.40995607
|
1586.40614067
|
|
H
|
0.00000000
|
0.00000000
|
0.00000000
|
0.00000000
|
0.00000000
|
20.78600000
|
|
Ar
|
0.00000000
|
0.00000000
|
0.00000000
|
0.00000000
|
0.00000000
|
20.78600000
|
|
H2O
|
0.14904476
|
-5.60452380
|
81.54220310
|
-573.43988184
|
1954.64102748
|
-2558.72544088
|
|
OH
|
0.03478011
|
-1.49678745
|
23.95819609
|
-180.33447646
|
643.43907081
|
-844.81094064
|
|
CO2
|
0.09995132
|
-2.84698619
|
29.97175824
|
-139.36548820
|
262.46393332
|
-77.60476812
|
|
CO
|
0.22518502
|
-7.60385762
|
101.08195929
|
-660.23411744
|
2118.61214474
|
-2644.11606414
|
|
NO
|
0.22148616
|
-7.36223109
|
96.15006747
|
-615.33775005
|
1927.66396650
|
-2333.02836259
|
|
NO2
|
0.19152294
|
-5.86302804
|
69.10109523
|
-388.90870179
|
1041.04770615
|
-1021.47920094
|
Table 1: ai coefficients
for 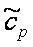 (T) logarithmic polynomial ( (T) logarithmic polynomial (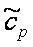 =[J/(mol K)]). T range 100-5000 K =[J/(mol K)]). T range 100-5000 K
|
Species
|
b6
|
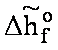 [J/mol] [J/mol]
|
c0
|
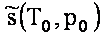 [J/(mol K)] [J/(mol K)]
|
|
CO
|
7800.122163
|
-110530
|
2599.24460
|
197.54
|
|
CO2
|
-9799.444922
|
-393520
|
-272.97823
|
213.69
|
|
H2O
|
6509.129599
|
-241830
|
2604.14357
|
188.72
|
|
H2
|
-17906.36645
|
0
|
-1741.84295
|
130.57
|
|
OH
|
-3897.017179
|
38990
|
774.89000
|
183.6
|
|
O2
|
-6650.197901
|
0
|
174.43449
|
205.04
|
|
O
|
-2214.908597
|
249170
|
653.63407
|
160.95
|
|
H
|
-6197.3459
|
218000
|
-118.43025
|
114.61
|
|
N2
|
7785.831947
|
0
|
2599.39565
|
191.5
|
|
NO
|
4728.18155
|
90290
|
2188.40197
|
210.65
|
|
N
|
2102.605182
|
472690
|
1259.03834
|
153.19
|
|
Ar
|
-6197.3459
|
0
|
-118.43025
|
154.74
|
|
NO2
|
-5094.0761401
|
33100
|
643.35865
|
239.92
|
Table 2 : Constants of integration, enthalpy of formation and
entropy at standard temperature and pressure (T0=298.15 K; p0=101325
Pa).
bi coefficients
For 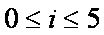 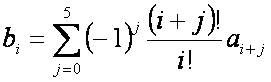 with with 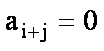 if if 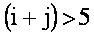 . .
ci coefficients
For 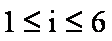 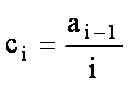
gi coefficients
For 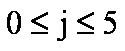 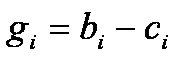 ; ; 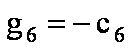 ; ; 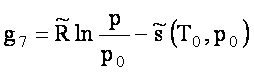 ; ; 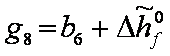 . .
FUELS
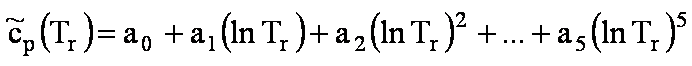
where Tr=T/Tc, and Tc is the critical
temperature.
Gases
|
a5
|
a4
|
a3
|
a2
|
a1
|
a0
|
Tc [K]
|
|
CH4
|
1.113859542417
|
-8.126750445589
|
15.025961612383
|
8.352335756601
|
-2.404135926621
|
33.795315991848
|
190.6
|
|
H2
|
-0.550917399284
|
8.017500998798
|
-44.303786812909
|
116.180997229773
|
-142.745181228842
|
92.723369415269
|
33.18
|
|
C2H2
|
-0.685785450427
|
2.518552060825
|
-2.583752519696
|
-0.393383689077
|
21.146593031886
|
44.657822823590
|
308.3
|
|
C2H6
|
1.457419131544
|
-6.417868237502
|
-2.216122356576
|
26.864672365436
|
37.425406759034
|
53.402669829242
|
305.3
|
|
C2H4
|
0.869739220990
|
-3.711682191020
|
-2.638789345297
|
19.484575858130
|
26.129392212759
|
41.822396850196
|
282.5
|
|
C3H6
Propylene
|
1.428847247677
|
-4.532044443762
|
-7.920453792757
|
22.974005052290
|
56.985504816355
|
75.100379950903
|
365.2
|
|
C4H8
Isobutene
|
1.865344616292
|
-5.062391642773
|
-12.213362041771
|
24.386936189875
|
84.426210096985
|
113.383421571580
|
417.9
|
|
C6H6
Benzene
|
1.635491384823
|
1.874523186055
|
-19.525673274541
|
-4.962242141294
|
112.384804738456
|
151.973495753845
|
562
|
|
C2H5OH
Ethanol
|
1.406449620188
|
-1.706745625638
|
-12.273943910039
|
10.791673475246
|
66.913660398690
|
97.301090949513
|
514
|
|
C2H6O Dimethyl
Ether
|
1.741753260725
|
-5.279712512663
|
-7.892002067064
|
22.526711137421
|
52.198156726227
|
78.806477323085
|
401
|
|
CH3OH Methanol
|
1.252049265734
|
-2.354757843251
|
-8.560257334851
|
12.844477210231
|
39.101511197012
|
60.606728470937
|
513
|
|
|
|
|
|
|
|
|
|
C3H8
Propane
|
2.574463389529
|
-6.108276774152
|
-11.646243309120
|
29.352775907335
|
72.860102727303
|
88.057699295447
|
369.8
|
|
C4H10
n-Butane
|
4.081926973040
|
-6.014749912413
|
-20.404917243700
|
30.695600769273
|
103.165833594102
|
130.809320121216
|
425
|
|
C4H10
Isobutane
|
4.172373106751
|
-6.102997248632
|
-20.254921490994
|
29.874057392009
|
105.058301457939
|
126.391862206308
|
407.7
|
|
C6H12
Cyclohexane
|
8.663061219494
|
0.424827359963
|
-50.753681958148
|
10.233541509341
|
190.647385669939
|
208.068597432136
|
554
|
|
C8H10
Ethylbenzene
|
7.423756470282
|
9.296388299484
|
-35.063301754915
|
-14.498955815686
|
165.102631065286
|
241.097002083027
|
617
|
|
(CH3)2CO Acetone
|
2.558004187811
|
-2.112206476350
|
-16.494167681729
|
14.714491484398
|
75.903738746707
|
109.164509352917
|
508
|
|
|
|
|
|
|
|
|
|
C7H8
Toluene
|
0.232332728211
|
5.800974440390
|
-22.456596375320
|
-9.356103392090
|
138.096042867477
|
194.430789869627
|
593
|
|
C5H12
n-Pentane
|
5.971767165123
|
-2.893108938525
|
-32.331240603903
|
29.518376457842
|
137.781247187080
|
173.788694897066
|
469.7
|
|
C7H16
n-Heptane
|
8.272402992493
|
6.627828947469
|
-45.597895797777
|
10.611329615533
|
194.117084022317
|
266.863703069824
|
540
|
|
C8H18
n-Octane
|
6.514781099349
|
9.573211425471
|
-47.927505630343
|
0.866570314137
|
219.241682396858
|
314.987178863040
|
568.9
|
|
C8H18
(2,2,4-Trimethyl
pentane)
|
4.975658204206
|
-1.101165006849
|
-32.943169193235
|
22.340443163095
|
231.245787291135
|
312.674401159893
|
543.9
|
|
C6H14
n-Hexane
|
13.127616255887
|
5.119027362386
|
-45.935938792348
|
11.854919521635
|
170.525103989822
|
231.325598244493
|
507.6
|
Table 3: ai
coefficients for 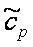 (T) logarithmic polynomial ( (T) logarithmic polynomial (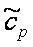 =[J/(mol K)]; =[J/(mol K)];
T range 200-3000 K).
|